Engineers and scientists at Rice University have developed a sweet way for petrochemical refineries to transform a smelly byproduct into cash.
Hydrogen sulfide gas has the distinct odor of rotting eggs. It frequently emanates from sewers, stockyards, and landfills, but it is especially problematic for refineries, petrochemical plants, and other industries. Thousands of tons of the noxious gas are produced annually as a byproduct of processes that remove sulfur from petroleum, natural gas, coal, and other products in these places.
Naomi Halas, a Rice engineer, physicist, and chemist, and colleagues describe a process that uses gold nanoparticles to convert hydrogen sulfide into sulfur and high-demand hydrogen gas in a single step in a study that was recently published in the journal ACS Energy Letters. Even better, the one-step process only needs light as its source of energy. Co-authors of the study include Hossein Robatjazi of Syzygy Plasmonics, Emily Carter of Princeton University, and Peter Nordlander of Rice University.
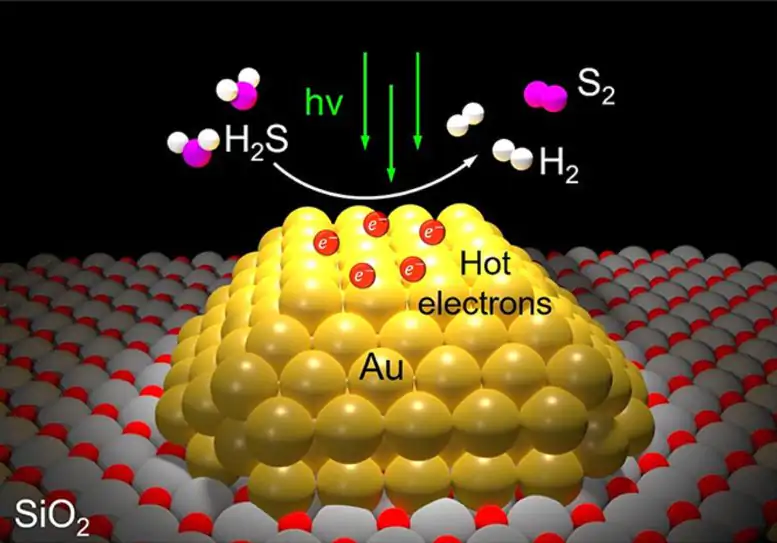
An illustration of the light-powered, one-step remediation process for hydrogen sulfide gas made possible by a gold photocatalyst created at Rice University. Credit: Halas Group/Rice University
“Hydrogen sulfide emissions can result in hefty fines for industry, but remediation is also very expensive,” said Halas, a nanophotonics pioneer whose lab has spent years developing commercially viable light-activated nanocatalysts. “The phrase ‘game-changer’ is overused, but in this case, it applies. Implementing plasmonic photocatalysis should be far less expensive than traditional remediation, and it has the added potential of transforming a costly burden into an increasingly valuable commodity.”

Rice University’s Naomi Halas is an engineer, chemist, physicist, and pioneer in the field of light-activated nanomaterials. Credit: Jeff Fitlow/Rice University
Each molecule of hydrogen sulfide gas (H2S) contains two hydrogen atoms and one sulfur atom. Each molecule of clean-burning hydrogen gas (H2), the primary commodity of the hydrogen economy, contains two hydrogen atoms. Halas’ team sprinkled the surface of grains of silicon dioxide powder with tiny islands of gold in the new study. Each island was a gold nanoparticle 10 billionths of a meter in size that interacted strongly with a certain wavelength of visible light. These plasmonic reactions create “hot carriers,” which are short-lived, high-energy electrons capable of driving catalysis.
In the study, Halas and co-authors used a laboratory setup and showed a bank of LED lights could produce hot carrier photocatalysis and efficiently convert H2S directly into H2 gas and sulfur. That’s a stark contrast to the established catalytic technology refineries use to break down hydrogen sulfide. Known as the Claus process, it produces sulfur but no hydrogen, which it instead converts into water. The Claus process also requires multiple steps, including some that require combustion chambers heated to about 1,500 degrees Fahrenheit.
The plasmonic hydrogen sulfide remediation technology has been licensed by Syzygy Plasmonics, a Houston-based startup company with more than 60 employees, whose co-founders include Halas and Nordlander.
Halas said the remediation process could wind up having low enough implementation costs and high enough efficiency to become economical for cleaning up nonindustrial hydrogen sulfide from sources like sewer gas and animal wastes.
“Given that it requires only visible light and no external heating, the process should be relatively straightforward to scale up using renewable solar energy or highly efficient solid-state LED lighting,” she said.
Reference: “Direct H2S Decomposition by Plasmonic Photocatalysis: Efficient Remediation plus Sustainable Hydrogen Production” by Minghe Lou, Junwei Lucas Bao, Linan Zhou, Gopal Narmada Naidu, Hossein Robatjazi, Aaron I. Bayles, Henry O. Everitt, Peter Nordlander, Emily A. Carter and Naomi J. Halas, 30 September 2022, ACS Energy Letters.
DOI: 10.1021/acsenergylett.2c01755
The study was funded by the Welch Foundation, the Air Force Office of Scientific Research, and the Defense Threat Reduction Agency.
On October 3, Halas and Nordlander were presented the prestigious 2022 Eni Energy Transition Award in recognition of their efforts to develop efficient light-powered catalysts for industrial-scale hydrogen production.
Halas is Rice’s Stanley C. Moore Professor of Electrical and Computer Engineering and a professor of chemistry, bioengineering, physics and astronomy, and materials science and nanoengineering. Nordlander is Rice’s Wiess Chair and Professor of Physics and Astronomy, and professor of electrical and computer engineering, and materials science and nanoengineering. Carter is Princeton’s Gerhard R. Andlinger Professor in Energy and Environment at the Andlinger Center for Energy and the Environment, senior strategic advisor for sustainability science at the Princeton Plasma Physics Laboratory, and professor of mechanical and aerospace engineering and of applied and computational mathematics. Robatjazi is chief scientist at Syzygy Plasmonics and an adjunct professor of chemistry at Rice.



